
Immuno-Oncology
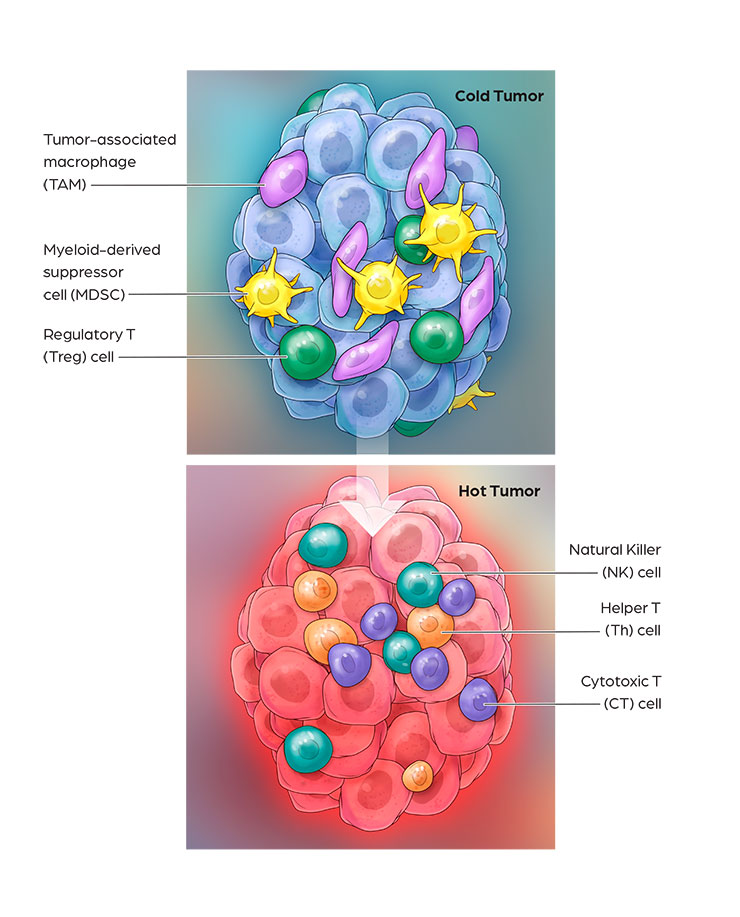
Cancer immunotherapy has demonstrated impressive clinical benefits in the fight against cancer by harnessing innate and adaptive immune responses. Many solid tumors are resistant to immunotherapies such as PD-1 and CTLA4 blockade, due to low T cell infiltration, insufficient neoantigen presentation, and immuno-suppression. Activation of these “cold tumor” cells via TLR7/8 agonists is a promising approach to overcome resistance mechanisms to current cancer immunotherapies. CanWell is building its immuno-oncology product platform with 2 lead programs: 1) CAN1012, a highly selective TLR7 agonist and 2) DPV-001, a dendritic cell-targeted cancer vaccine.
CAN1012
CAN1012 is a novel, highly selective TLR7 agonist that activates plasmacytoid DCs. In pre-clinical studies, CAN1012 has shown high anti-tumor immune responses, including proinflammatory cytokine production and the priming of cytotoxic T-lymphocyte (CTL) responses. Compared with other TLR7/8 drugs currently under clinical development, CAN1012 has demonstrated several important differentiating properties, including better solubility, higher potency, longer tissue retention and minimal systemic toxicity. CAN1012 selectively induces efficacy-favorable cytokines and TH1 immune response (increasing CD4 & CD8, decreasing MDSC & M2), and reduces induction of toxicity-related cytokines. CAN1012 is not expected to induce cytokine tolerance under multiple dosing and its anti-tumor effects can be enhanced through combination with checkpoint inhibitors such as anti PD/L-1, anti-CTLA4, etc. Phase I clinical study of CAN1012 in advanced cancer patients is ongoing in both US and China.DPV-001
Developed by UbiVac, an US cancer vaccine pioneer, DPV-001 is a first-in-class cancer vaccine that exploits autophagy to educate the immune system and further destroy cancer cells. DPV-001 is a dendritic cell-targeted microvesicle containing short-lived proteins that are thought to represent the dominant HLA-presented epitopes on the surface of cancer cells. When used in combination with other immunotherapies such as immune checkpoint inhibitors and T cell agonists, DPV-001 significantly augments anticancer immunity in preclinical models. DPV-001 is currently being evaluated in multiple US clinical Ph1b/Ph2 studies by our partner UbiVac for the safety, tolerability, and preliminary efficacy in patients with advanced tumors. In February 2021, CanWell entered into an exclusive license with UbiVac for commercial development of DPV-001 in Greater China. By leveraging the encouraging US clinical data generated by UbiVac, CanWell is launching its first Ph1 clinical trials in China.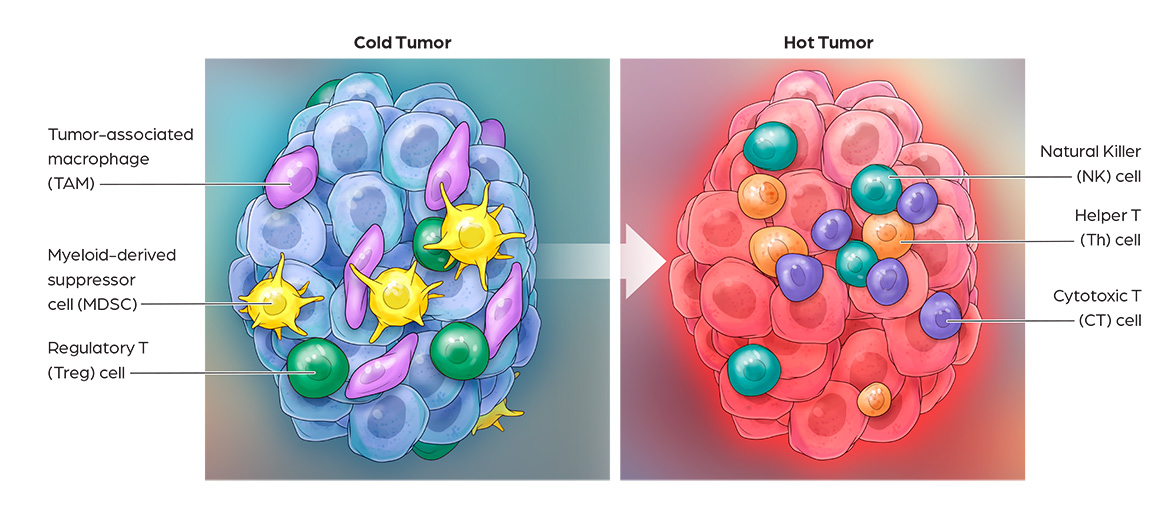
Small Molecule & Drug Conjugate Platform
Chemotherapy remains the current first-line treatment for many different cancers. As a systemic medication, chemotherapy drugs travel through the bloodstream and reach all parts of the body, they can also damage healthy cells. So toxicity and systemic side effects of chemotherapy always limit optimal treatment outcomes. By applying its novel chemical conjugation technology, CanWell has developed several small molecule drug candidates with improved therapeutic windows and significantly better safety profiles.
CAN2109
CAN2109 is a novel long-acting immunogenic cell death (ICD) agent with unique duel anti-tumor mechanisms of action, including robust immune modulating and direct cell killing. Preclinical studies have shown that CAN2109 eliminates tumors with a single IT injection. By establishing long-term immune memory against tumor cells, those treated with CAN2109 are less susceptible to tumor re-attack. The anti-tumor activities of CAN2109 were found to be dependent on CD4 and CD8 T cells. In November 2023, CAN2109 received FDA approval for Phase I clinical trials.Antibody Drug Conjugate Platform
StarLinker Technology Platform
It is always desirable to have ADCs with higher DARs to allow more effective cell tumor killing, and there is also an increasing interest in developing the next generation of ADCs with dual cytotoxic agents from different MOAs to produce synergistic effects. CanWell has developed a novel StarLinker technology platform where each linker loads up to 4 units of therapeutic agents. It offers significant flexibility in generating ADCs with DARs ranging from 4 to 32. More importantly, it has the capability to load 2 or more different therapeutic agents at different ratios. Preclinical studies have shown the ADCs generated under StarLinker platform have good hydrophilicity, little aggregation, and satisfactory PK profile. StarLinker has the potential to become an important enabling technology in the development of the next generation ADCs with high DARs and/or dual/multi-drug ADCs.
TLR ADC (Immune-Stimulating Antibody Conjugate)
TLR agonists have shown encouraging antitumoral activities in early clinical trials, but challenges remain in developing next generation TLR-based therapies with robust immune stimulating potencies and satisfactory safety profiles. Based on our proprietary TLR molecule library, CanWell has developed several TLR-ADC conjugates by using TLR agonists as ADC payloads, which have different TLR7 or 8 selectivity. Preclinical studies strongly demonstrated proof-of-concept results on molecule selectivity, chemistry conjugation, stability, in vivo PK, and efficacy in mouse tumor models.